

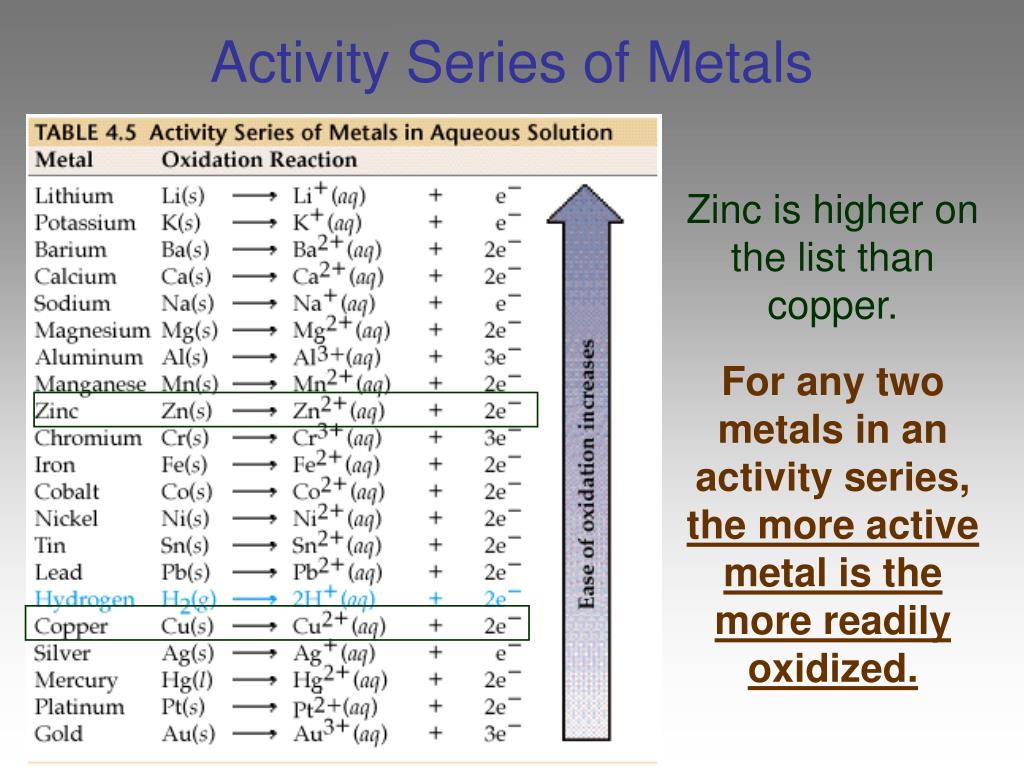
Therefore, designing high-performance Pt electrocatalysts with low Pt dosage and high durability is extremely desirable for alkaline HOR, which should be the stone of killing two birds (i.e., low abundance of Pt element and unsatisfactory alkaline HOR performance).īy deciphering the alkaline HOR process 2, 17, 18, 19, it is found that the mutual competition in the adsorption-desorption between reaction intermediates (e.g., *H and *OH) and CO on the surface-active sites of electrocatalysts is the key reason for the low catalytic activity and poor CO tolerance 20, 21, 22.
However, the large-scale implementation of conventional Pt/C electrocatalysts in alkaline HOR catalysis is beset by ultralow catalytic efficiency (e.g., two orders of magnitude decline), extortionate Pt dosage (e.g., ten times higher than that in acidic media), and poor durability in terms of weak CO tolerance and feeble stability 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. Hydrogen fuel cells as green yet high-efficiency energy suppliers are very attractive in contributing to fulfilling carbon neutrality, which is greatly benefited from the deployment of precious Pt electrocatalysts performing best in catalyzing anodic hydrogen oxidation reaction (HOR) due to the optimal adsorption/desorption energy toward hydrogen intermediates 1, 2, 3. This work may shed light on the design of metal nanocluster-based electrocatalysts for energy conversion. Mechanism studies reveal that oxophilic single-atom lanthanide species in Pt nanoclusters can serve as the Lewis acid site for selective OH - adsorption and regulate the binding strength of intermediates on Pt sites, which promotes the kinetics of hydrogen oxidation and CO oxidation by accelerating the combination of OH − and *H/*CO in kinetics and thermodynamics, endowing the electrocatalyst with up to 14.3-times higher mass activity than commercial Pt/C and enhanced CO tolerance. Herein we report the design of a series of single-atom lanthanide (La, Ce, Pr, Nd, and Lu)-embedded ultrasmall Pt nanoclusters for efficient alkaline hydrogen electro-oxidation catalysis based on vapor filling and spatially confined reduction/growth of metal species. Designing Pt-based electrocatalysts with high catalytic activity and CO tolerance is challenging but extremely desirable for alkaline hydrogen oxidation reaction.


 0 kommentar(er)
0 kommentar(er)
